|
CHENGDU, China, Oct. 18, 2025 /PRNewswire/ — Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd. (the “Company”) announced that at the 2025 European Society for Medical Oncology (ESMO) Congress held in Berlin, Germany, results from a Phase 3 OptiTROP-Breast02 study of the Company’s trophoblast cell-surface antigen 2 (TROP2)-directed antibody-drug conjugate (ADC) sacituzumab tirumotecan (sac-TMT) (佳泰莱®) in previously treated locally advanced or metastatic hormone receptor-positive (HR+) and human epidermal growth factor receptor 2 -negative (HER2-) breast cancer (BC) was presented as an oral report by Professor Man Li from the Second Affiliated Hospital of Dalian Medical University (Presentation # LBA23, Proffered paper session 1: Breast cancer, metastatic). Previously, the new indication applications for sac-TMT for this indication was accepted by the National Medical Products Administration (NMPA) and was included in the priority review and approval process.
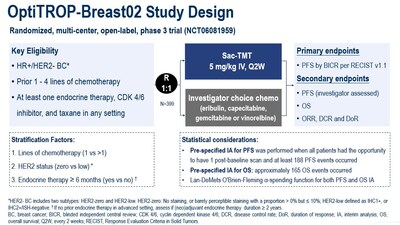
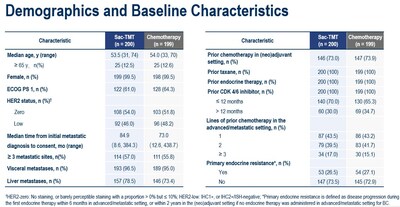
In the OptiTROP-Breast02 study, a total of 399 patients with HR+/HER2- BC who had progression on CDK4/6 inhibitors and received at least one prior line of chemotherapy in the advanced/metastatic setting were randomized (1:1) to receive sac-TMT or investigator’s choice of chemotherapy (ICC).
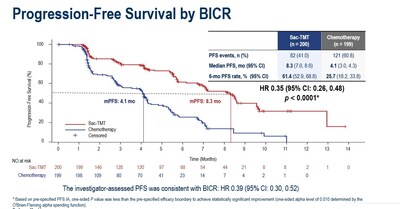
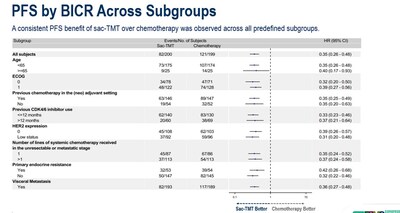
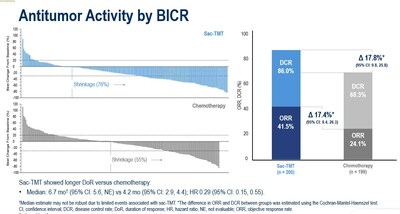
As at the data cut-off date January 22, 2025, the median PFS was significantly longer in sac-TMT than in ICC (8.3 vs. 4.1 months; HR, 0.35; 95% CI, 0.26-0.48; P<0.0001). Clinical benefit was seen in sac-TMT independent of HER2 expression (HR for PFS in HER2-zero: 0.39, 95% CI, 0.26-0.57; in HER2-low: 0.31, 95% CI, 0.20-0.48).
Sac-TMT showed longer DoR versus chemotherapy; and ORR was also superior with sac-TMT to ICC.
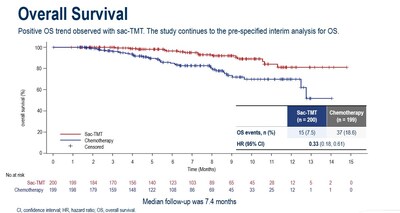
There was a trend in OS that favored sac-TMT over ICC (HR, 0.33; 95% CI, 0.18-0.61).
Grade ≥ 3 TRAEs occurred in 62.0% and 64.8% of patients in sac-TMT and ICC. TRAE led to discontinuation in 0% and 0.5% of patients; pneumonitis occurred in 1.5% and 1.0% of patients (all grade 1-2) in sac-TMT and ICC, respectively.
As a conclusion, sac-TMT demonstrated statistically significant and clinically meaningful improvement in PFS compared to chemotherapy. PFS benefit was observed across all predefined subgroups and independent of HER2 status. Sac-TMT also showed a trend toward OS benefit (HR, 0.33; 95% CI, 0.18, 0.61) and demonstrated a favorable and manageable safety profile, with no new safety signals. Currently, a Phase III clinical study of sacituzumab tirumotecan as monotherapy and in combination with pembrolizumab in patients with chemotherapy-naïve HR+/HER2- breast cancer is ongoing globally, with a planned enrollment of approximately 1,200 participants. Additionally, another Phase III registrational study in the same population is currently enrolling patients in China, with a planned enrollment of 430 participants.
Professor Man Li from the Second Affiliated Hospital of Dalian Medical University, stated:”The Phase III OptiTROP-Breast02 study confirmed that, regardless of HER2 expression status, sac-TMT demonstrated promising efficacy in previously treated HR+/HER2- BC patients. As the first Phase III clinical trial focusing exclusively on an all-Chinese population with HR+/HER2- BC, the presentation of the OptiTROP-Breast02 study data at ESMO marks a significant breakthrough in clinical research in this indications in China. The positive outcomes of this study not only provide evidence-based support for the treatment of HR+/HER2- BC but also offer a safe and reliable new treatment option for breast cancer patients.”
About sac-TMT (佳泰莱®)
Sac-TMT, a core product of the Company, is a novel human TROP2 ADC in which the Company has proprietary intellectual property rights, targeting advanced solid tumors such as NSCLC, BC, GC, gynecological tumors, among others. Sac-TMT is developed with a novel linker to conjugate the payload, a belotecan-derivative topoisomerase I inhibitor with a drug-to-antibody-ratio (DAR) of 7.4. Sac-TMT specifically recognizes TROP2 on the surface of tumor cells by recombinant anti-TROP2 humanized monoclonal antibodies, which is then endocytosed by tumor cells and releases the payload KL610023 intracellularly. KL610023, as a topoisomerase I inhibitor, induces DNA damage to tumor cells, which in turn leads to cell-cycle arrest and apoptosis. In addition, it also releases KL610023 in the tumor microenvironment. Given that KL610023 is membrane permeable, it can enable a bystander effect, or in other words kill adjacent tumor cells.
In May 2022, the Company licensed the exclusive rights to MSD (the tradename of Merck & Co., Inc, Rahway, NJ, USA) to develop, use, manufacture and commercialize sac-TMT in all territories outside of Greater China (which includes Mainland China, Hong Kong, Macao and Taiwan).
To date, three indications for sac-TMT have been approved and marketed in China for the treatment of adult patients with unresectable locally advanced or metastatic triple negative breast cancer (TNBC) who have received at least two prior systemic therapies (at least one of them for advanced or metastatic setting), EGFR mutation-positive locally advanced or metastatic non-squamous NSCLC following progression on EGFR-TKI therapy and platinum-based chemotherapy and EGFR mutant-positive locally advanced or metastatic non-squamous NSCLC who progressed after treatment with EGFR-TKI therapy. Sac-TMT is the first TROP2 ADC drug approved for marketing in lung cancer globally. In addition, the new indication applications for sac-TMT for the treatment of adult patients with unresectable locally advanced, metastatic HR+/HER2- BC who have received prior endocrine therapy and other systemic treatments in the advanced or metastatic setting was accepted by the Center for Drug Evaluation (CDE) of the National Medical Products Administration (NMPA), and was included in the priority review and approval process.
As of today, the Company has initiated 9 registrational clinical studies in China. MSD has initiated 15 ongoing Phase 3 global clinical studies of sac-TMT as a monotherapy or with pembrolizumab or other anti-cancer agents for several types of cancer. These studies are sponsored and led by MSD.
About Kelun-Biotech
Kelun-Biotech (6990.HK) is a holding subsidiary of Kelun Pharmaceutical (002422.SZ), which focuses on the R&D, manufacturing, commercialization and global collaboration of innovative biological drugs and small molecule drugs. The company focuses on major disease areas such as solid tumors, autoimmune, inflammatory, and metabolic diseases, and in establishing a globalized drug development and industrialization platform to address the unmet medical needs in China and the rest of world. The Company is committed to becoming a leading global enterprise in the field of innovative drugs. At present, the Company has more than 30 ongoing key innovative drug projects, of which 4 projects have been approved for marketing, 1 project is in the NDA stage and more than 10 projects are in the clinical stage. The company has established one of the world’s leading proprietary ADC and novel DC platforms, OptiDC™, and has 2 ADC project approved for marketing and multiple ADC and novel DC assets in clinical or preclinical research stage. For more information, please visit https://kelun-biotech.com/.