|
SEOUL, South Korea, Aug. 26, 2025 /PRNewswire/ — Brightonix Imaging, a global leader in cutting-edge medical imaging technology, is proud to announce that its flagship product, the PHAROS PET Scanner, has received FDA clearance for commercial distribution in the United States. This milestone marks a new era of precision and efficiency in nuclear imaging and positions Brightonix Imaging at the forefront of medical innovation.
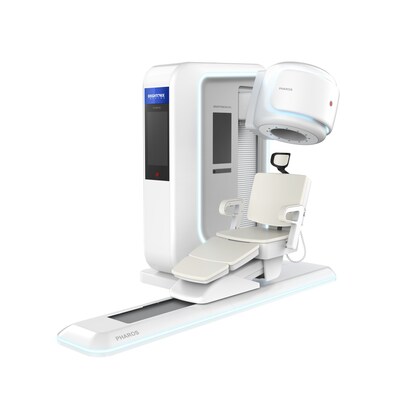
The PHAROS PET is a state-of-the art clinical positron emission tomography (PET) system designed to deliver exceptional image quality, offering healthcare providers a powerful tool for early disease detection, precise diagnosis, and optimized treatment planning. With its innovative design and enhanced performance capabilities, the PHAROS scanner is poised to set new standards in neurology. Furthermore, the PHAROS is multi-functional with the ability to physically orient the patient seat and detector configurations for extremity and breast imaging, as well as converting to both lying and seated modes for brain imaging.
Key Features and Benefits of the PHAROS PET Scanner:
- Unprecedented Image Clarity: Utilizing advanced detector technology, the PHAROS PET Scanner delivers high-resolution images, enabling clinicians to visualize even the most subtle anomalies for accurate diagnosis and treatment.
- Compact Footprint: The PHAROS scanner is designed with a space-efficient footprint, enabling easier installation in a wide range of clinical environments. Its streamlined design provides greater flexibility for hospitals and imaging centers with limited space, without compromising imaging performance.
- Flexible Applications: Its multifunctionality allows tailored configurations for brain, breast, and extremity PET scans, while offering a more comfortable setup that enhances the overall patient experience.
- User-Friendly Interface: Featuring an intuitive interface and streamlined workflow, the PHAROS PET Scanner is designed to be easily operated by clinicians and technologists, enhancing user experience and ensuring seamless integration into medical practices.
Prof Jae Sung Lee, CEO and Founder at Brightonix Imaging, said, “Receiving FDA clearance for the PHAROS PET Scanner is a monumental achievement for us and for the entire medical imaging community. This technology will empower healthcare providers with the tools to detect and treat neuro degenerative diseases earlier and with greater precision, ultimately improving patient outcomes.”
The development of PHAROS was supported by the Korea Medical Device Development Fund (KMDF), reflecting a collaborative effort across multiple government agencies to advance innovative medical imaging technologies.
About Brightonix Imaging
Founded with a commitment to innovation and excellence in medical imaging, Brightonix Imaging develops cutting-edge solutions that enhance diagnostic accuracy, patient care, and overall clinical performance. The company’s portfolio includes advanced clinical PET, medical AI software and preclinical PET/CT imaging systems. Brightonix is committed to driving the future of biomedical imaging with products that are both technologically advanced and accessible.
Next Steps
With FDA clearance secured, Brightonix Imaging will begin the process of rolling out the PHAROS PET Scanner to medical facilities across the United States. Interested healthcare providers and institutions can contact Brightonix Imaging for demonstrations, product details, and ordering information. For more information about the PHAROS PET Scanner and other products, please visit www.brtnx.com.